Forging New Frontiers in Cancer Immunotherapy
We are advancing existing immunotherapy with novel allosteric anti-PD-1 antibodies to help improve therapeutic strategies in oncology. Our lead candidate, MAQ-001, allosterically regulates the PD-1 immune checkpoint inhibitor through a unique allosteric mechanism—paving the way for a new immunomodulation paradigm and more effective, durable combination therapies
Unlocking New Therapeutic Pathways

The first decade of PD-1/PD-L1 immune checkpoint inhibitor antibody therapies has revolutionized cancer treatment, with six lead therapies achieving blockbuster success. Despite response rates of 30–45% in melanoma and NSCLC, many patients with other cancers remain underserved due to resistance and limited options. In addition, the median duration of the response and progression-free survival are still measured in months rather than years. Traditional PD-1/CTLA-4 immunotherapy combinations show modest improvements in efficacy, but are hampered by severe toxicity, leading to treatment discontinuation in up to 35% of patients.
MabQuest’s allosteric PD-1 novel mechanistic approach offers an alternative solution with the potential to significantly overcome resistance observed in PD-L1 low cancers observed with orthosteric anti-PD-1 antibodies, thereby improving efficacy in a wide range of cancers and prolonging durable responses - charting a path to address critical unmet needs in cancer therapy.
Redefining PD-1 Modulation
Our novel class of anti-PD-1 antibodies target a unique allosteric site on the PD-1 receptor that is distinct from and non-overlapping with the site bound by classical anti-PD-1 antibodies currently approved for cancer immunotherapy. By targeting this novel site, we fine-tune immune activation and restore the tumor-fighting capabilities of exhausted CD8 T cells while limiting off-target immune related adverse events.
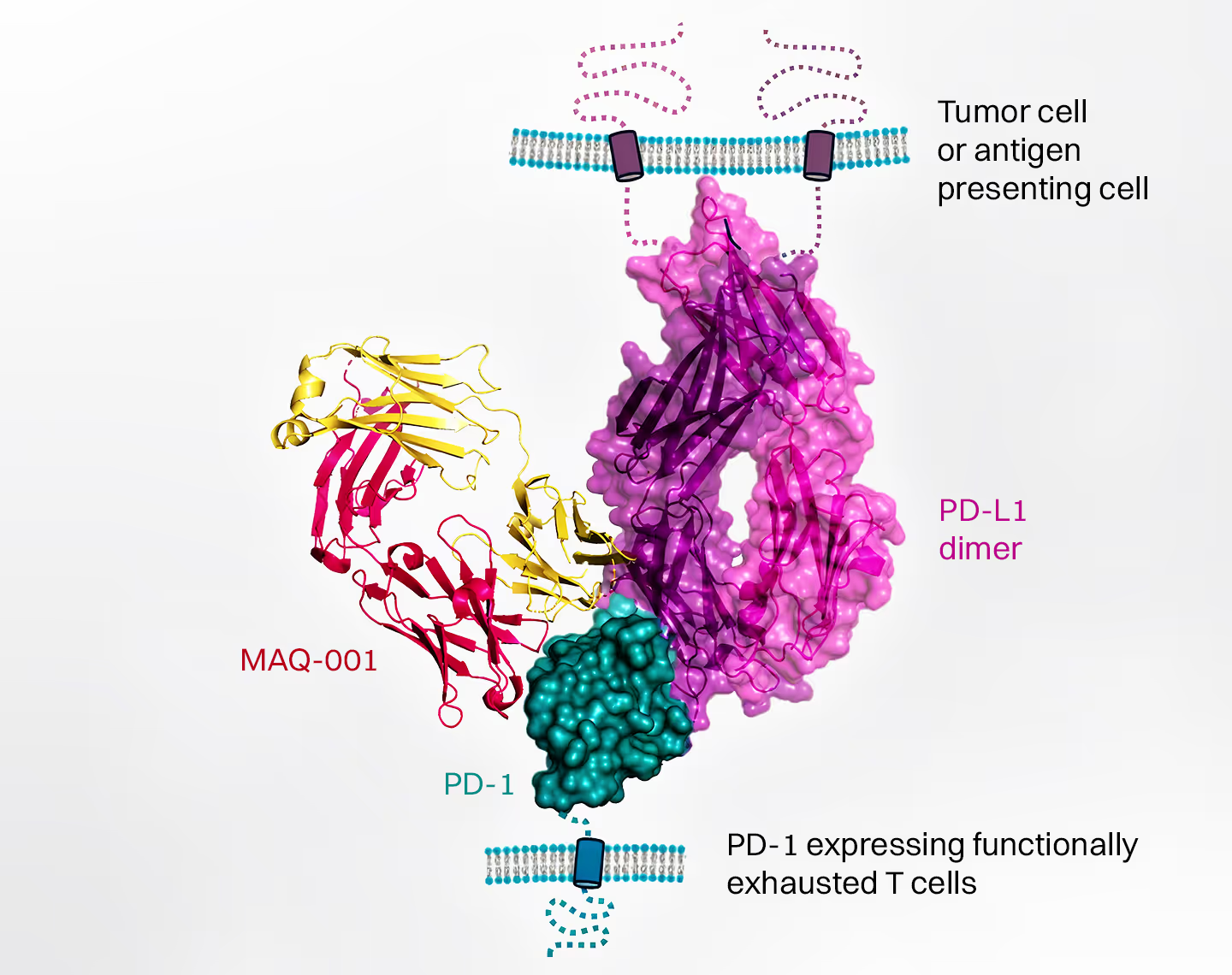
Innovative Mechanism
MAQ-001 prevents PD-1 from effectively suppressing the CD28 co-receptor, which acts through the NFκB pathway to provide important positive signals to increase the survival and proliferation of tumor-attacking immune cells. Unlike traditional therapies that block PD-L1 binding to PD-1, our approach relieves T cell exhaustion through an allosteric mechanism independent of PD-L1 binding, and early clinical data shows evidence of reduced off-target toxicity while demonstrating anti-tumor activity.
Combination Potential
MAQ-001’s unique mechanism of action complements existing therapies by synergizing with classical blocking anti-PD-1 antibodies to unlock the full potential relief from the suppressive effects of the PD-1 immune checkpoint inhibitor. It also provides enhanced combination activity with various targeted therapies, including anti-CTLA-4 therapies.
Comparison with Conventional Therapies
Classical anti-PD-1 therapies that block receptor binding have been overwhelmingly successful in cancer therapy by preventing PD-L1 mediated sequestration of PD-1 and the associated phosphatase SHP-2 into contact with the TCR and CD28 T cell activation complex. While this partially restores cancer-specific T cell activation, our allosteric strategy primarily targets the CD28 pathway associated with survival and proliferation, which may preserve critical immune functions, resulting in a safer and more effective treatment alternative that can benefit a broader range of patients including those with resistant or complex cancers.
Advancing Allosteric Innovations: Our Oncology Pipeline
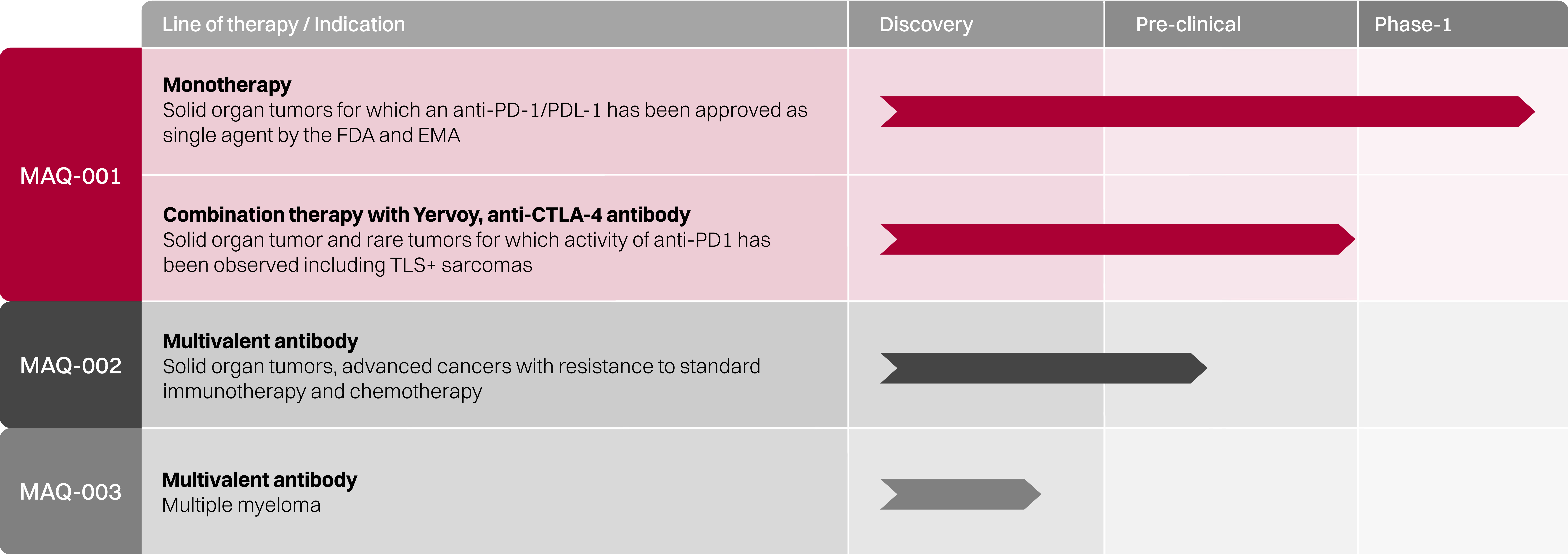
Built on our novel allosteric anti-PD-1 mechanism, MabQuest’s comprehensive oncology pipeline is driven by strong preclinical evidence and a clear roadmap to clinical development.
MAQ-001
Our lead candidate has demonstrated anti-tumor activity equivalent to approved anti-PD-1 therapies, such as Keytruda, in preclinical animal models in vivo. Furthermore, phase 1a clinical trials have shown that MAQ-001 is well tolerated and shows preliminary evidence of anti-tumor activity in humans.
MAQ-002
MabQuest is advancing a second novel allosteric monoclonal antibody in a bispecific format, intended to widen the spectrum of cancers we target with greater efficacy. Stay tuned for more MAQ-002 updates as it advances through preclinical evaluation and prepares for clinical development.
Peer-Reviewed Innovation
Our platform for antibody drug discovery is validated by a robust portfolio of peer-reviewed research. Key publications in journals such as Journal of Experimental Medicine, Nature Microbiology, Journal of Infection, Cell Reports, Cell Host and Microbe and Frontiers in Immunology underscore our commitment to scientific excellence and innovation in immune checkpoint modulation.